MAKING A KILLING: Pfizer continues push for COVID-19 vaccine boosters despite lack of late-stage clinical trial results
08/16/2021 / By Nolan Barton
Pfizer CEO Albert Bourla recently expressed confidence that a booster would increase the immunity levels of people who already received two doses of the company’s coronavirus (COVID-19) vaccine.
“We are very, very confident that a third dose, a booster, will take up the immune response to levels that will be enough to protect against the delta variant,” Bourla said.
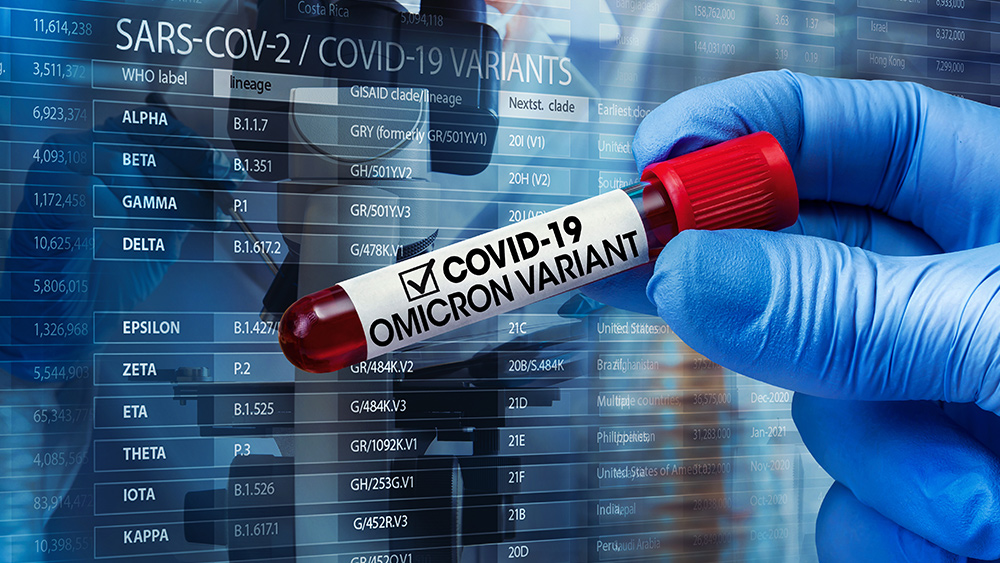
Unfortunately, Pfizer has not yet delivered conclusive proof to back up Bourla’s confidence. The company lacks late-stage clinical trial results to confirm a booster will work against COVID variants, including delta that now accounts for 93 percent of new infections across the U.S.
The pharmaceutical giant announced its global phase 3 trial on a third dose in mid-July. That trial’s completion date is in 2022. Phase 3 results generally are required before regulatory approval.
Drug regulators not sold on COVID-19 vaccine boosters
Pfizer and partner BioNTech said last month that they plan to ask U.S. and European regulators to authorize a booster dose of its COVID-19 vaccine based on evidence of greater risk of infection six months after vaccination and the spread of the highly contagious delta variant. (Related: Big Pharma companies begin push for coronavirus booster shots, with no end in sight.)
But the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) said in a joint statement that Americans who have been fully vaccinated do not need a booster at this time. The European Medicines Agency (EMA), on the other hand, said it was confident for now that the established regimen was sufficient.
At the end of July, Pfizer and BioNTech announced findings that their vaccine’s efficacy dropped to about 84 percent four to six months after a second dose.
Preliminary data in Israel published in the same month found that the Pfizer-BioNTech Covid-19 vaccine was just 40.5 percent effective on average at preventing symptomatic disease.
The data also appeared to show a waning effectiveness of the Pfizer-BioNTech vaccine. It was only 16 percent effective against symptomatic infection for those who had two doses back in January. The efficacy rate against symptomatic infection stood at 79 percent for those who had received two doses by April.
A new preprint study by the Mayo Clinic this month also found that the vaccine’s effectiveness against infection dropped to 42 percent from January to July.
“We are confident in this vaccine and the third dose, but you have to remember the vaccine efficacy study is still going on, so we need all the evidence to back up that,” said Jerica Pitts, Pfizer’s director of global media relations.
If a third dose couldn’t combat the delta or other variants, Pfizer said it will come up with a “tailor-made” vaccine within 100 days. Pfizer and BioNTech announced they are developing an updated version of their vaccine in Germany to target the genomic features of the delta variant.
However, the idea that a new formulation could work better is “mostly hypothetical at this point,” said Vaughn Cooper, a professor of microbiology and molecular genetics at the University of Pittsburgh.
Big Pharma’s rush to recommend boosters frustrate health experts
The pharmaceutical industry’s rush to recommend boosters for the public is “a little frustrating,” said Dr. Paul Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia and an adviser to the National Institutes of Health (NIH) and the FDA.
In any case, decisions about boosters do not rest with vaccine makers. “Pharmaceutical companies aren’t public health agencies, it’s really not theirs to determine when or whether there should be booster dosing,” Offit said.
Dr. Sadiya Khan, an epidemiologist and cardiologist at Northwestern University Feinberg School of Medicine, said that the push for giving boosters to healthy populations is premature. That’s because even if those already fully vaccinated do get a third dose or booster, the virus is still circulating among millions of unvaccinated people.
“Giving up on that greater strategy of vaccinating the population is going to lead to continued surges,” she said. “The potential for harm is quite large.”
The White House has added to the mixed messaging. Spokesperson Jen Psaki confirmed that the U.S. will buy an additional 200 million doses of the Pfizer-BioNTech vaccine for children under 12 and for possible boosters. (Related: Even the WHO says booster shots are unnecessary, but Biden’s White House prefers to listen to Big Pharma: BOOSTER covid shots coming to the USA.)
The confusion has set off a feverish search for an illicit third dose just in case it’s necessary.
“I snuck in a dose of Pfizer last week,” Angie Melton, a 50-year-old mother of four, shared on Facebook. Melton received the one-dose Johnson & Johnson vaccine at a mass vaccination site in April and feared the highly contagious delta variant could infect her and then her unvaccinated 10-year-old son, who has asthma.
“I’m trying to keep my family safe,” Melton said.
Vaccine expert warns governments that vaccinations fuel spread of dangerous variants
Vaccine expert Dr. Geert Vanden Bossche had seen this coming. In March, he wrote an open letter warning world leaders and health officials that vaccinations will fuel the spread of new “dangerous variants” of the virus.
In the letter, Vanden Bossche urged governments to stop vaccination drives. He said that mass vaccinations are “likely to further enhance adaptive immune escape as none of the current vaccines will prevent replication or transmission of viral variants.” (Related: Fully vaccinated Americans are SPREADING covid’s delta variant, health expert warns.)
Immune escape is a term used to describe when the host is no longer able to recognize and counter a pathogen, such as a relevant variant or mutant of SARS-CoV-2 – the virus that causes COVID-19.
“This type of prophylactic vaccines is completely inappropriate, and even highly dangerous, when used in mass vaccination campaigns during a viral pandemic,”
A prophylactic or preventative vaccine involves introducing antigens into a person’s body. The goal is that the individual’s immune system will create antibodies for those antigens and become immune to the associated illness.
“The more we use these vaccines for immunizing people in the midst of a pandemic, the more infectious the virus will become,” Vanden Bossche wrote. “With increasing infectiousness comes an increased likelihood of viral resistance to the vaccines.”
Under this scenario, manufacturers will be forced to refine or improve the vaccines, which will then increase the selection pressure. Selection pressure is a term used to describe the process that helps an organism or pathogen to evolve in ways that make it better adapted to its changing environment. An antibiotic resistance, which is caused by overuse of antibiotic drugs, is a good example of selection pressure.
In other words, the virus will effectively outsmart the highly specific antigen-based vaccines that are being used and tweaked.
Follow Immunization.news for more news and information related to coronavirus vaccines.
Sources include:
Tagged Under: bad medicine, Big Pharma, boost shots, CDC, clinical trial, coronavirus, covid variants, covid-19, COVID-19 vaccine, Delta Variant, FDA, immunity level, outbreak, pandemic, pharma profits, SARS-CoV-2, science fraud, spike protein, symptomatic disease, symptomatic infection, vaccination drive, vaccine wars, vaccine's efficacy, vaccines
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 CDC NEWS